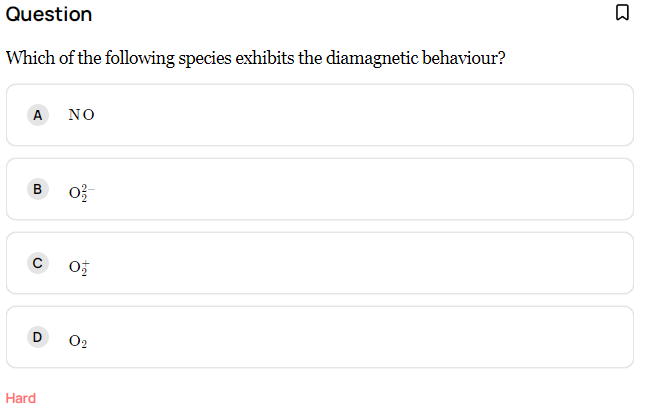We are here with another amazing trick of chemistry that is to find that the given compound is Diamagnetic or Paramagnetic. You can see various question directly come from this topic in JEE, NEET, Boards that make this trick very important to learn.
Before going to the trick see these questions that are asked in exams-
By the help of this trick you will be able to find the given compound is diamagnetic or paramagnetic, it will take hardly 5 to 10 seconds to solve!
This question was asked in JEE and categorized as hard but it is possible to get the answer within 5 seconds!
Paramagnetic or Diamagnetic Trick:-
- Step 1:- Find total number of electron in given species. (e.g. For NO = 7+8 = 15)
- Step 2:- If it is a odd number, 10, 16 or 32 then it is a paramagnetic species.
- Step 3:- It sum is other than this than it is diamagnetic species.
- Example: no. of electrons in NO are 15 that is odd so NO is paramagnetic.
More Examples:-
O2 :- no. of electrons = 8+8=16 so it is Paramagnetic.
S2:- no. of electrons = 16+16=32 so it is Paramagnetic.
N2:- no. of electrons = 7+7=14 so it is diamagnetic.
Read more tricks:-
Tags:-
how to calculate paramagnetic and diamagnetic how to determine diamagnetic and paramagnetic how to identify paramagnetic compounds how to know if a compound is paramagnetic or diamagnetic how to know if diamagnetic or paramagnetic most paramagnetic paramagnetic and diamagnetic trick paramagnetic diamagnetic trick paramagnetic diamagnetic trick calculation